Weight-loss drugs among breakthrough trials offering ‘hope’ for dementia prevention
One clinical trial is assessing whether semaglutide, used in weight loss drugs, could slow the progression of dementia

Dozens of breakthrough drug trials, including one using the main ingredient in weight-loss jabs, offer patients “hope” for the treatment and prevention of dementia and Alzheimer’s disease.
Some 138 treatments are being assessed in clinical trials in 2025 - an 11 per cent rise on the year before - according to new research published on Tuesday.
Experts have said that 2025 could be a “landmark year” in breakthrough medicines as four drugs that could prevent the disease, not just treat it, have progressed to large clinical trials.
Some trials include drugs currently used to treat other conditions. One such trial is assessing whether semaglutide, the main ingredient for the weight loss and diabetes drugs Ozempic and Wegovy, can slow the progression of dementia.
An estimated 982,000 people are living with dementia in the UK, and this is projected to rise to 1.4 million in 2040, according to the Alzheimer’s Society.
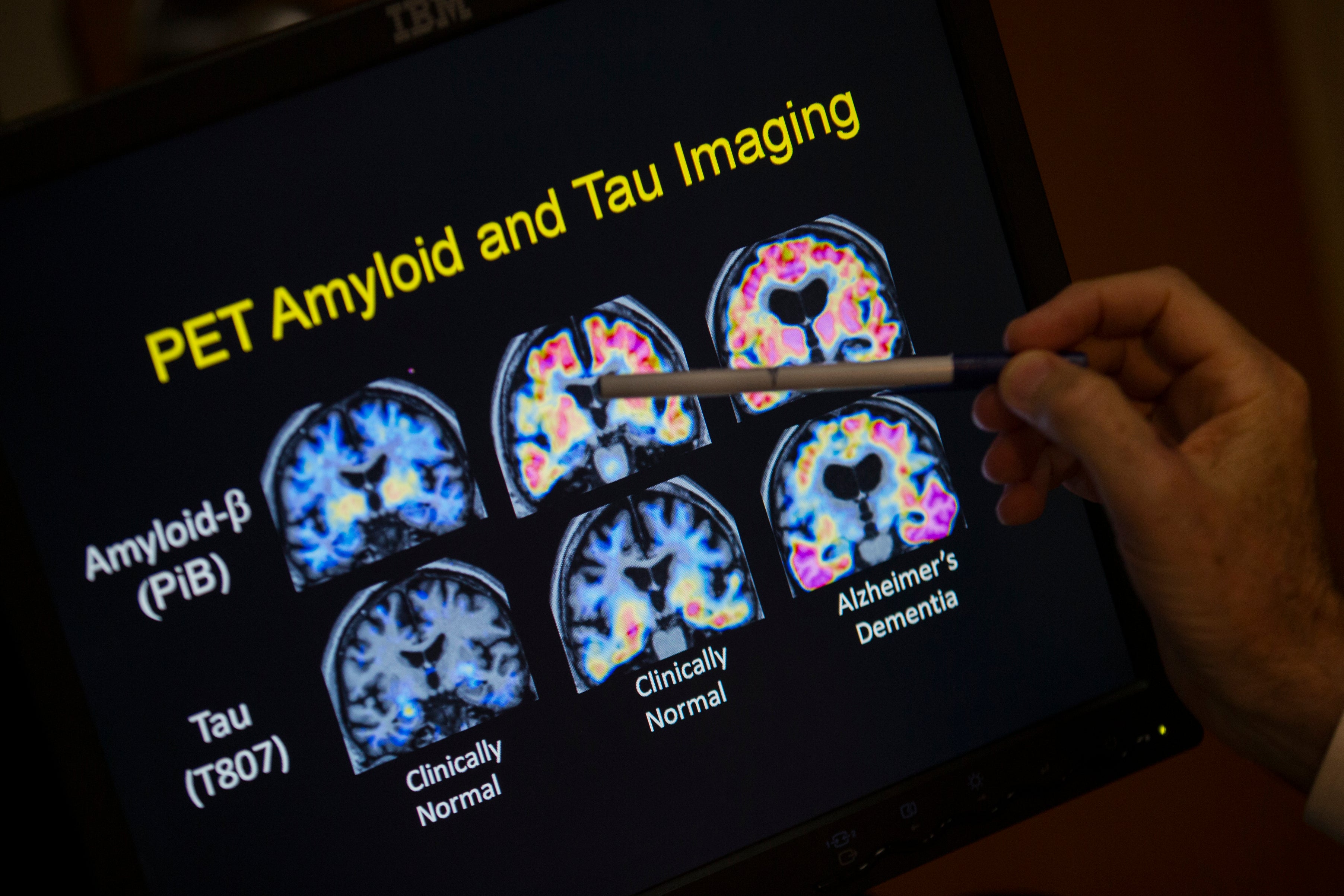
Dr Emma Mead, chief scientific officer of the Oxford Drug Discovery Institute, added: “Today we are at a tipping point in dementia research as we understand more and more about the diseases that drive dementia.
“This gives us opportunities to slow and ultimately stop this devastating condition, and today’s announcement demonstrates that researchers are able to translate these understandings towards potential new treatments.”
Dr Richard Oakley, associate director of research at Alzheimer’s Society, said: “2025 is shaping up to be a landmark year for Alzheimer’s disease drug development.
“With more trials underway than ever before and more drugs entering the pipeline, there is hope on the horizon for the nearly one million people living with dementia in the UK.”
A report from the University of Nevada in the US and published in the journal Alzheimer’s and Dementia: Translational Research and Clinical Interventions, said a “robust” range of drugs was being trialled in 2025 and a “notable increase” showed “momentum toward identifying new therapies for the treatment of alzheimer’s disease”.
Experts said that drugs, such as such as lecanemab and donanemab, that target amyloid protein build-up in the brain - a key characteristic of both diseases - are “only one part of the overall strategy” as they expressed excitement over the variety of new drugs being tested.
James Rowe, professor of cognitive neurology at the University of Cambridge and consultant neurologist, said: “One of the most exciting things of this report is the number of large-scale late-stage trials on prevention. And the aspiration to prevent, not just treat, is starting to be seen in the figures we see in these charts today.”
He added: “The ones that are in trial at the moment are really… bringing forward an effective treatment to earlier stage.”
For instance, people with a genetic risk of Alzheimer’s could receive some drugs earlier to see if they protect them against developing the disease.
Dr Sheona Scales, director of research at Alzheimer’s Research UK, said that, as well as more drugs coming through the pipeline, the treatment targets are “more diverse” and “looking at all stages of the disease”.
She added: “What this paper is showing us is that the pipeline of drug development is growing, it’s diversifying and accelerating.”
Meanwhile, academics said lecanemab and donanemab, which can be used for treating mild cognitive impairment in Alzheimer’s patients, are an “important first step” in the battle against the disease.
The treatments were initially approved for UK use by regulators but then deemed not cost-effective for NHS use.
The National Institute for Health and Care Excellence (NICE) is taking more evidence on donanemab and lecanemab and is expected to announce its decision in the summer.
Dr Scales added: “Lecanemab and donanemab have represented a huge leap forward in our understanding and ability to be able to treat Alzheimer’s disease.
“What they’ve done is they’ve proved that we’re able to modify the course of Alzheimer’s disease, and what that has done is opened up the door to future treatments that we hope are more effective, easier to deliver and and able to deliver for our patients.”
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments